Immunology News: Targeting Myeloid-Derived Suppressor Cells (MDSCs) for Cancer Immunotherapy
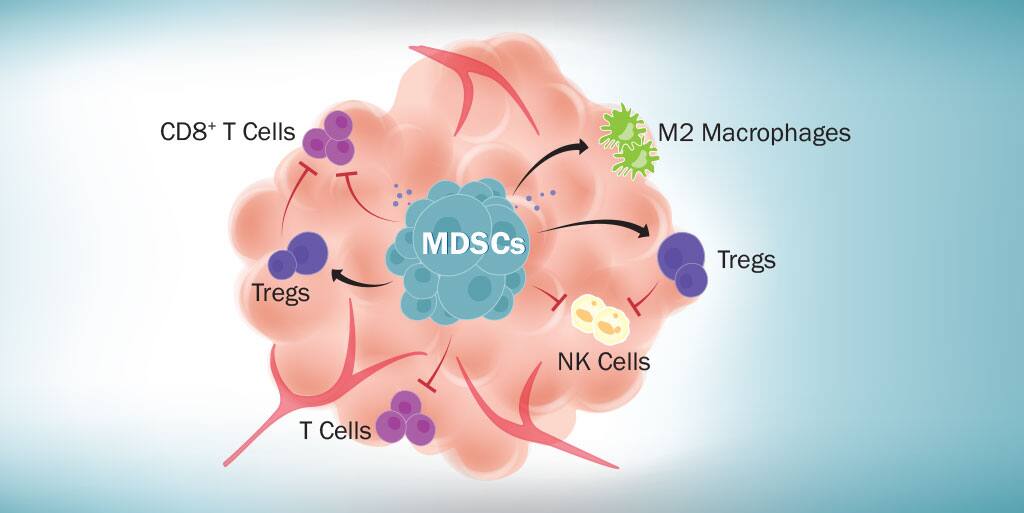
Myeloid-derived suppressor cells (MDSCs) are a diverse population of immature myeloid cells with immunosuppressive properties that accumulate under pathological conditions including specific types of cancer and infections. Two primary subsets of human and mouse MDSCs known as granulocytic and monocytic MDSCs have been identified. These cells are of great interest as high levels of circulating MDSCs in cancer patients have been shown to correlate with clinical stage, metastatic burden, and resistance to both chemotherapy and immunotherapy. As a result, MDSCs are now recognized as a major obstacle in the treatment of cancer. Multiple strategies targeting these cells are being investigated to determine if the immunosuppressive effects of MDSCs can be reduced or eliminated to improve the efficacy of anti-cancer treatments. Although no therapeutic drugs specifically targeting MDSCs have been approved to date, the effects of several agents intended to either deplete MDSCs, promote their differentiation, or inhibit their development, infiltration, or activity are being evaluated in tumor-bearing mice and cancer patients.
Under normal physiological conditions, MDSCs are not present in the circulation, but under pathological conditions, these cells accumulate. MDSC accumulation is mediated by two signals: 1) high levels of tumor- or niche-derived growth factors (GM-CSF, G-CSF, M-CSF, SCF, and VEGF) that promote the expansion of immature myeloid cells from the bone marrow and spleen and inhibit their terminal differentiation into macrophages, dendritic cells, or granulocytes and 2) the presence of pro-inflammatory cytokines (IFN-gamma, IL-1 beta, IL-4, IL-6, IL-13, and TNF-alpha) that promote the pathological activation of MDSCs. The effects of several agents that promote MDSC differentiation or those that block MDSC development are being investigated as potential therapeutic strategies to attempt to eliminate MDSCs in the tumor microenvironment. These include all-trans retinoic acid (ATRA), vitamin A, vitamin D3, IL-12, and CpG oligonucleotides to try to drive MDSC differentiation, and bisphosphonates, Jak/STAT inhibitors, VEGF inhibitors, and multi-kinase inhibitors to attempt to block MDSC development. Additional agents aimed at inhibiting the ability of MDSCs to infiltrate tumors are also being studied including anti-glycan antibodies designed to target the receptor for advanced glycation end products (RAGE) - S100A8/A9 feedback loop that is thought to be involved in promoting the recruitment of MDSCs to colon carcinomas, in addition to antibodies or inhibitors against IL-17, CCR2, CSF-1R, CXCR2, and galectin-3, which have all been suggested to play a role in MDSC recruitment to the tumor microenvironment.
In addition to agents that promote MDSC differentiation and those that block MDSC development or tumor infiltration, agents that inhibit the immunosuppressive functions of MDSCs are also being examined. MDSCs suppress immune cell functions through multiple mechanisms including expression of high levels of arginase 1 (ARG1), inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS), along with IL-10 and TGF-beta secretion. These molecules, either individually or together, allow MDSCs to inhibit T cell proliferation, promote T cell apoptosis, inhibit dendritic cell development, inhibit the functions of CD8+ T cells and natural killer (NK) cells, and stimulate regulatory T cell expansion. Several different arginase, nitric oxide, and ROS inhibitors are being evaluated to determine if any of these inhibitors can effectively suppress the activities of MDSCs in vivo and improve the clinical outcome for cancer patients. These include COX2 inhibitors and N-hydroxy-L-Arginine (NOHA), which inhibit ARG1 activity, Bardoxolone methyl (CDDO-Me), an inhibitor of ROS production, nitro-aspirin (NO-aspirin), a nitric oxide inhibitor, and N(G)-Nitro-L-Arginine Methyl Ester (L-NAME) and PDE-5 inhibitors, which function as both nitric oxide and ARG1 inhibitors. Most of these inhibitors have been tested in tumor-bearing mouse models with some showing considerable promise.
MDSC depletion strategies are also being investigated to attempt to eliminate the deleterious immunosuppressive effects of MDSCs in the tumor microenvironment. Research in this area has primarily focused on cytotoxic agents such as cisplatin, 5-fluorouracil, gemcitabine, and paclitaxel, which have all been reported to inhibit MDSC accumulation. Unfortunately, these agents can also have adverse side effects. Significantly, an article published earlier this year suggests a new strategy for MDSC depletion that is more selective. In a report published in Cell in February 2018, Tavazoie, et al. found that a liver X receptor (LXR) beta agonist could reduce both the intratumoral and peripheral granulocytic and monocytic MDSC populations in multiple mouse cancer models by increasing MDSC apoptosis. The authors demonstrated that this effect was mediated through the LXR target gene, ApoE, binding to the LRP8 receptor on the surface of MDSCs. Furthermore, they showed that the decrease in MDSCs was associated with an increase in tumor-associated CD8+ and CD4+ T cells. Using a mouse model of melanoma, the authors found that co-administration of an LXR agonist with tumor antigen-specific cytotoxic T lymphocytes (CTL) significantly enhanced CTL activity, reduced tumor volume, and increased mouse survival when compared to mice receiving melanoma tumor antigen-specific CTL therapy alone. Similarly, using mouse models of melanoma or lung cancer, they demonstrated that co-administration of the LXR agonist with anti-PD-1 therapy significantly increased the number of tumor-infiltrating, activated cytotoxic T cells and enhanced anti-tumor activity when compared with anti-PD-1 therapy alone. These findings are particularly exciting as they not only describe a new method by which MDSCs can be selectively depleted from the tumor microenvironment and show that LXR activation is associated with an increase in CD8+ T cell activation in cancer patients with a variety of different tumor types, but that LXR activation can also improve the anti-tumor response in current immunotherapy models. Combined with the authors’ previous observations that LXR agonism could suppress melanoma tumor progression, angiogenesis, invasion, and metastasis, this report suggests that the positive effects of LXR activation may be far-reaching as it may have the potential to inhibit tumor growth and metastasis in a range of different cancers.
As immunosuppressive factors in the tumor microenvironment are now recognized as a major obstacle for cancer immunotherapy, there is significant interest in identifying these factors and finding ways to inhibit their activities. MDSCs accumulate in the tumor microenvironment and suppress the functions of multiple different cell types that are critical for the anti-tumor immune response. As a result, researchers are currently evaluating the effects of several MDSC targeting agents with the hope of being able to develop new therapeutics that will improve the efficacy of current cancer immunotherapy strategies.
References
Tavazoie, M.F. et al. (2018) Cell 172:825.
Additional resources related to this topic:
MDSC-Mediated Mechanisms of Immunosuppression Pathway